Research and Treatment
A large part of Alliance to Cure Cavernous Malformation’s mission is to drive research for better treatments and a cure. At this time, more than ten possible treatments have been identified and three are in clinical trials.
Our current work is a multi-faceted effort to engage treatment developers, to create and maintain shared tools and resources, and to assist researchers in their patient recruitment and retention efforts, to support efforts to develop expanded methods of measuring drug efficacy.
1) Accelerating Cures Initiative
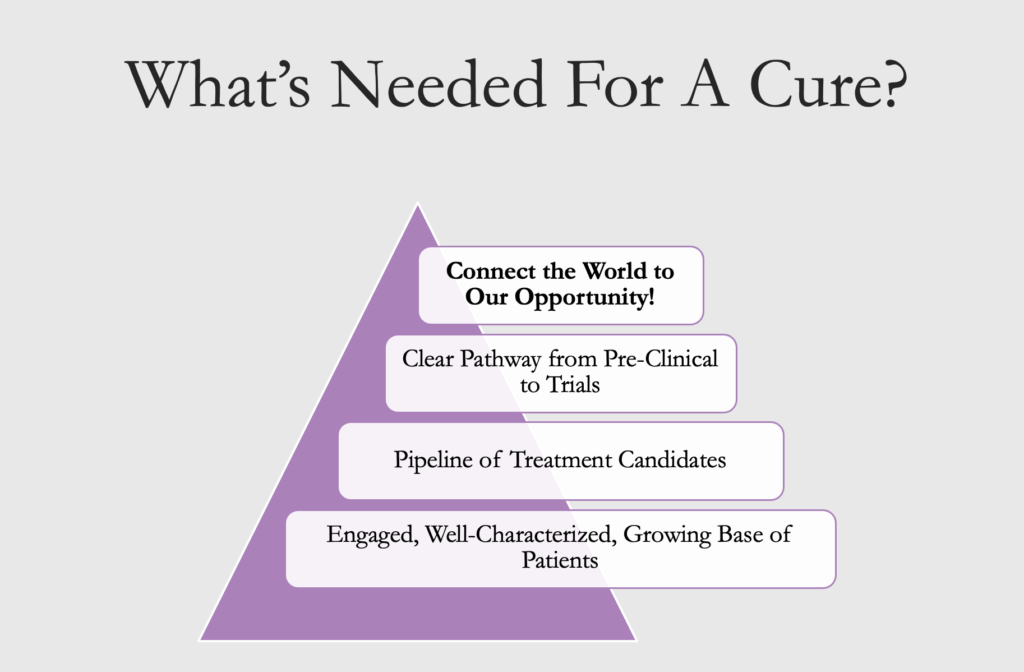
It has taken researchers and the patient community years to develop all the pieces needed for a successful drug trial. Within the next few years, we will have everything in place: a robust and eager patient community, tools like validated surveys and biomarkers that will measure the efficacy of a medicine, a network of Centers of Excellence to host trials, and a large pipeline of potential treatments. We now need to attract the attention of treatment developers (the pharmaceutical industry and others) in what is a very competitive field among rare diseases. We need to convey the message that we are ready, that a drug trial is feasible, and that developing treatments for CCM would bring a good return on investment.
To that end, in 2021, we launched an initiative to accelerate cures. We hired an Industry Relations Director to coordinate a critical examination of our current resources in order to address any deficiencies, craft a white paper delineating our strong position, and engage in outreach to prospective industry partners.
We have begun to fund research that moves beyond pills to treat cavernous malformation to explore avenues that may offer actual cures. Our Research You Are Funding page is updated with our latest projects and currently includes focused ultrasound, gene therapy, targeted medication delivery, and mRNA treatment.
The project also includes launching bridge research that covers any gaps between academic work and the requirements of industry. One example of such research is a mouse study to offer a head-to-head comparison of the effectiveness of treatments under consideration. We may also be asked to participate in funding pilot trials of treatments that are not under patent. In 2022, we launched this project by creating a shared database that researchers may use to record their mouse data – the mouse models they have in their labs and the outcomes of the treatments they’ve tested. If we are asked to fund pilot trials, the cost for any project would be $400,000 in annual salary and bridge research.
2) Cavernous Malformation Patient Registry
Our patient registry was upgraded in March 2023 to provide easier access for researchers and better data collection. As of this writing, we have over 3000 patients enrolled. We are pushing to find as many affected individuals as possible willing to share their information. This data helps us understand the illness and what our members can offer research and trials. The patient registry forms the foundation for recruiting for any clinical study, including upcoming drug trials. Moving forward, it will help researchers to design better trials. The registry costs include platform costs, costs to program new surveys, staff for outreach and data analysis, and advertising and promotional expenses. The patient registry and the associated research efforts are an annual $98,000 annual expense.
Research Efforts Supported by the Patient Registry:
Clinical Drug Trial Recruitment and Retention – With clinical trials and additional clinical research underway, the patient registry is used daily to identify individuals who may qualify as participants and share information about participation opportunities. We identify and contact potential participants individually and assess their eligibility. We have four years of trial experience and are expanding our role by performing regular follow-ups to encourage compliance and retention.
Data Analysis to Shape Trials – We work closely with pharmaceutical companies and academics to create viable trial designs and accompany them to formal FDA meetings. We allow direct access to anonymous registrant data to researchers. We also offer an analysis of data as requested by treatment developers.
CCM-Health Index – The CCM-Health Index is a tool under development to measure the impact of the illness on the lives of our members. Such a measure will be critical in determining whether a medication benefits our families. Alliance to Cure Cavernous Malformation is in an academic/industry partnership to create a survey that has required rounds of recruiting – up to 500 registrants in total – to participate in the development. This was no small task – it has meant one-on-one outreach with at least half of the participants, requiring hundreds of hours of staff time. Also, because of the registry, we can be active participants in the FDA qualification process, allowing the tool to be used as a clinical endpoint in trials. In fact, in 2019, Alliance to Cure Cavernous Malformation initiated an FDA Listening Session in which registry data and patient stories were used to convey the patient experience of the illness to FDA officials. This meeting paved the way for Recursion’s REC-994 Phase II trial plan. We will continue to recruit for this project until the CCM-Health Index receives FDA qualification as an acceptable clinical trial endpoint, with a target of 2022.
Microbiome Project and Non-Pharmaceutical Interventions – One of the more exciting lines of inquiry for our members is research related to non-pharmaceutical interventions that may impact the progression of the disease. Research is ongoing in the following areas: Vitamin D supplementation, microbiome research exploring the impact of gut bacteria and compromised gut lining integrity in the illness, and hypoxia/sleep apnea. Studies are also exploring the potential negative effects on cavernous malformation lesions of hormone use and treating migraine with Botox injections. Alliance to Cure Cavernous Malformation has supported this research by enrolling patients in pilot studies that have generated data leading to breakthrough findings and publications. Larger projects are beginning, and Alliance to Cure Cavernous Malformation will play a large role in promoting participation among our members and conveying research results to the patient population. We disseminate information about research findings to registrants in patient-friendly language, including creating a CCM-healthy cookbook that is in its 2nd edition and hosting multiple webinars and discussion groups.
 COVID-19 Response – We have created a separate COVID-19 registry to track individuals with CCM who also have tested positive for COVID-19. Because COVID-19 appears to result in increased clotting and vasculitis, there has been concern that our CCM patients would experience increased CCM activity if infected. We have also been concerned that those with familial CCM disease, in which every cell in the body has a CCM mutation, may experience more severe COVID-19 complications or may be more likely to become COVID-19 “long haulers.” Alliance to Cure Cavernous Malformation is the only organization or institution with the reach to capture an international cohort of patients to track their experience with COVID-19. This registry resulted in a paper in the Journal of Cerebrovascular Disease and Stroke describing the potentially higher risk of brain hemorrhage in those with sporadic CCM.
COVID-19 Response – We have created a separate COVID-19 registry to track individuals with CCM who also have tested positive for COVID-19. Because COVID-19 appears to result in increased clotting and vasculitis, there has been concern that our CCM patients would experience increased CCM activity if infected. We have also been concerned that those with familial CCM disease, in which every cell in the body has a CCM mutation, may experience more severe COVID-19 complications or may be more likely to become COVID-19 “long haulers.” Alliance to Cure Cavernous Malformation is the only organization or institution with the reach to capture an international cohort of patients to track their experience with COVID-19. This registry resulted in a paper in the Journal of Cerebrovascular Disease and Stroke describing the potentially higher risk of brain hemorrhage in those with sporadic CCM.
3) Annual Scientific Meeting
Our annual international CCM scientific meeting is the most important event each year for research progress. It is the only time our group of scientists from 5 continents meets face-to-face to share their unpublished research. This is the conference where they form new collaborations and map out a path to treatments. Several multi-institution research consortia use the opportunity to hold satellite meetings the day before the official start of our meeting. Every CCM scientific advance made in the last 15 years can attribute at least part, if not all, of its origin to our meeting. While our 2020 and 2021 meetings were virtual, we hosted an in-person meeting in November 2022 and will host another in July 2023. Building and maintaining our strong community of researchers requires personal interactions that can only be achieved face-to-face. The scientific meeting is an annual $50,000 expense.
4) DNA and Tissue Bank
Our DNA and Tissue Bank holds surgically-removed cavernous malformation tissue and accompanying DNA samples from our members. This is a resource used by researchers around the world. In 2022, we expanded the DNA and Tissue Bank to include other biological samples, including plasma, serum, and urine, which have become important in developing blood biomarkers. Expanding the DNA and Tissue Bank will require an annual $50,000 expense.
5) Expansion of our CCM Clinical Center Network
Alliance to Cure Cavernous Malformation has facilitated the development and recognition of CCM Centers of Excellence and CCM Clinical Centers to improve care and expand research. We refer our patients to these centers, knowing they will receive informed, coordinated care that follows our peer-reviewed Clinical Care Consensus Guidelines. These are also future locations for multi-center clinical drug trials. We are quickly expanding the CCM Clinical Centers network to support clinical drug trials. We are also developing CME materials to educate medical providers inside and outside of the network. Expanding the CCM Clinical Center Network and creating CME is an annual $140,000 expense, including staff time, travel, and materials.
6) Genetic Testing Program
Alliance to Cure Cavernous Malformation offers free genetic testing to any US or Canadian resident who cannot obtain insurance coverage for testing and has multiple cavernous malformations without an alternate explanation. Patients, clinicians, and researchers consider this one of our most important services because it helps with clinical decision-making and it prepares these patients to participate in research. Alliance to Cure Cavernous Malformation employs a licensed professional to order testing, and the test results are transmitted to the patient’s physician. Genetic testing is an annual $45,000 expense.

Patient Support and Information
The first half of our mission statement is “to inform, support, and empower those affected by cerebral cavernous malformation.” No one with cavernous malformation should feel alone. Everyone with cavernous malformation should be able to make informed decisions about their care.
1) CCM Connect Patient Navigation
We have launched a patient navigation program to connect our US-based members with resources beyond their initial CCM medical care. Surveys of our families indicate their primary needs are connections to second opinions, aftercare, and clinical trials. We are offer individualized consultations with warm handoffs to Centers and other resources. We have also added a natural language chatbot to our website to allow members to obtain information more easily. Our Patient Navigation budget is $75,000.
2) National Patient Conference
We host two types of patient conferences: national and regional. Our national conference is a multi-day event held in conjunction with the International Scientific Meeting. This allows the researchers to meet the individuals they are helping. The conferences include presentations by 5-8 experts, support groups, and social time. If the budget allows, children’s activities are also offered. The presentations from these conferences are live-streamed and available on YouTube. A national conference budget is approximately $20,000. Our regional conferences are hosted by Centers of Excellence. We support these with Alliance to Cure staff.
3) Family Camp
We hosted our first family camp in 2024 and, with appropriate resources, hope to host another in the coming years. Camp is an opportunity for our affected children and their siblings to meet other kids and for affected adults to find support. The first camp was life-changing for adults and kids alike. Family camp is a $30,000 expense.

Updated 3.05.25

